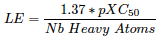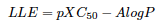Compound 984
Identifiers
- Canonical SMILES:
O=C(O)[C@H](Cc1ccccc1)N1C(=S)S\C(=C/c2ccc(OCCOCCOc3ccc(cc3)\C=C3/SC(=S)N(C3=O)[C@H](C(=O)O)Cc3ccccc3)cc2)C1=O
- IUPAC name:
(2S)-2-[(5Z)-5-[[4-[2-[2-[4-[(Z)-[3-[(1S)-1-carboxy-2-phenylethyl]-4-oxo-2-sulfanylidene-1,3-thiazolidin-5-ylidene]methyl]phenoxy]ethoxy]ethoxy]phenyl]methylidene]-4-oxo-2-sulfanylidene-1,3-thiazolidin-3-yl]-3-phenylpropanoic acid
- InChi:
InChI=1S/C42H36N2O9S4/c45-37-35(56-41(54)43(37)33(39(47)48)23-27-7-3-1-4-8-27)25-29-11-15-31(16-12-29)52-21-19-51-20-22-53-32-17-13-30(14-18-32)26-36-38(46)44(42(55)57-36)34(40(49)50)24-28-9-5-2-6-10-28/h1-18,25-26,33-34H,19-24H2,(H,47,48)(H,49,50)/b35-25-,36-26-/t33-,34-/m0/s1
- InChiKey:
HKIIFTOBMULLCQ-WKAHBFOZSA-N
External links
 44456329 |
 CHEMBL270646 |
 23324074 |
External search

|

|

|

|

|
Bibliography (1)
| Publication | Name |
|---|---|
| Wang L, Kong F, Kokoski CL, Andrews DW, Xing C. . Development of dimeric modulators for anti-apoptotic Bcl-2 proteins. Bioorganic & medicinal chemistry letters. | D3 |
Pharmacological data
| Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|
| 3 | 0 | 0 | 0 |
Targets
| PPI family | Best activity | Diseases | MMoA |
|---|---|---|---|
| BCL2-Like / BAX | 5.66 | cancer | Inhibition |
Physicochemical filters
| Descriptor | Lipinski's RO5 | Veber | Pfizer's 3/75 | |
|---|---|---|---|---|
| Compliance | ||||
| MW | 840.13 g/mol | |||
| HBA | 11 | |||
| HBD | 2 | |||
| HBA + HBD | 13 | |||
| AlogP | 8.80 | |||
| TPSA | 142.91 | |||
| RB | 18 |
Radar chart
PCA : iPPI-DB chemical space
PCA : Correlation circle
Efficiencies: iPPI-DB biplot LE versus LLE
Summary
| Bibliographic ressources | Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|---|
| 1 | 3 | 0 | 0 | 0 |
Pharmacological data
| Bibliography | Name | Target | Competition | Assay type | Assay name | Cell line | Activity type | Activity |
|---|---|---|---|---|---|---|---|---|
| 18023349 | D3 | BCL2 P10415 |
|
Biochemical assay | Fluorescence Polarization | pKi (inhibition constant, -log10) | 5.37 | |
| 18023349 | D3 | B2CL1 Q07817 |
|
Biochemical assay | Fluorescence Polarization | pKi (inhibition constant, -log10) | 5.64 | |
| 18023349 | D3 | B2CL2 Q92843 |
|
Biochemical assay | Fluorescence Polarization | pKi (inhibition constant, -log10) | 5.66 |
| Ta | Structure | Name | Drugbank ID |
|---|---|---|---|
| 0.5722 | Epalrestat | DB15293 | |
| 0.4937 | (E)-3-(5((5-(4-CHLOROPHENYL)FURAN-2-YL)METHYLENE)-4-OXO-2-THIOXOTHIAZOLIDIN-3-YL)PROPANOIC ACID | DB08177 | |
| 0.4887 | (Z)-3-BENZYL-5-(2-HYDROXY-3-NITROBENZYLIDENE)-2-THIOXOTHIAZOLIDIN-4-ONE | DB07838 | |
| 0.4866 | [(5R)-5-(2,3-dibromo-5-ethoxy-4-hydroxybenzyl)-4-oxo-2-thioxo-1,3-thiazolidin-3-yl]acetic acid | DB06998 | |
| 0.4466 | (5E)-5-[(2,2-DIFLUORO-1,3-BENZODIOXOL-5-YL)METHYLENE]-1,3-THIAZOLIDINE-2,4-DIONE | DB07503 | |
| 0.4105 | 4-{(1E)-3-OXO-3-[(2-PHENYLETHYL)AMINO]PROP-1-EN-1-YL}-1,2-PHENYLENE DIACETATE | DB08753 | |
| 0.4076 | Ponesimod | DB12016 | |
| 0.4024 | Carindacillin | DB09319 | |
| 0.3957 | Carfecillin | DB13506 | |
| 0.3938 | N-[(2S)-3-Phenyl-2-sulfanylpropanoyl]-L-phenylalanyl-L-tyrosine | DB03949 | |
| 0.3919 | Amoxicillin | DB01060 | |
| 0.3850 | (2R,4S)-2-[(1R)-1-{[(2R)-2-Amino-2-(4-hydroxyphenyl)acetyl]amino}-2-oxoethyl]-5,5-dimethyl-1,3-thiazolidine-4-carboxylic acid | DB03658 | |
| 0.3842 | N-Caffeoyltyramine | DB08754 | |
| 0.3816 | {4-[(2S)-2-({[(1S)-1-Carboxy-2-phenylethyl]carbamoyl}amino)-3-oxo-3-(pentylamino)propyl]phenoxy}malonic acid | DB02436 | |
| 0.3796 | Benzylpenicillin | DB01053 |