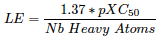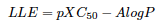Compound 2284
Identifiers
- Canonical SMILES:
CCC(N1[C@@H]([C@H](C[C@](C)(CC(O)=O)C1=O)c1cccc(Cl)c1)c1ccc(Cl)cc1)c1ccccn1
- IUPAC name:
2-[(3R,5R,6S)-5-(3-chlorophenyl)-6-(4-chlorophenyl)-3-methyl-2-oxo-1-[1-(pyridin-2-yl)propyl]piperidin-3-yl]acetic acid
- InChi:
InChI=1S/C28H28Cl2N2O3/c1-3-24(23-9-4-5-14-31-23)32-26(18-10-12-20(29)13-11-18)22(19-7-6-8-21(30)15-19)16-28(2,27(32)35)17-25(33)34/h4-15,22,24,26H,3,16-17H2,1-2H3,(H,33,34)/t22-,24?,26-,28-/m1/s1
- InChiKey:
WGSOZDLVXMXSND-URNXRWCWSA-N
External links
 168317835 |
 35S |
External search

|

|

|
|

|
Bibliography (1)
Pharmacological data
| Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|
| 1 | 1 | 0 | 0 |
Targets
| PPI family | Best activity | Diseases | MMoA |
|---|---|---|---|
| MDM2-Like / P53 | 8.52 | cancer | Inhibition |
Physicochemical filters
| Descriptor | Lipinski's RO5 | Veber | Pfizer's 3/75 | |
|---|---|---|---|---|
| Compliance | ||||
| MW | 510.15 g/mol | |||
| HBA | 5 | |||
| HBD | 1 | |||
| HBA + HBD | 6 | |||
| AlogP | 6.00 | |||
| TPSA | 73.33 | |||
| RB | 7 |
Radar chart
PCA : iPPI-DB chemical space
PCA : Correlation circle
Efficiencies: iPPI-DB biplot LE versus LLE
Summary
| Bibliographic ressources | Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|---|
| 1 | 1 | 1 | 0 | 0 |
Pharmacological data
| Bibliography | Name | Target | Competition | Assay type | Assay name | Cell line | Activity type | Activity |
|---|---|---|---|---|---|---|---|---|
| 10.1021/ml500142b | 6 | MDM2 Q00987 |
P53 P04637 |
Biochemical assay | HTRF | pIC50 (half maximal inhibitory concentration, -log10) | 8.52 | |
| 10.1021/ml500142b | 6 | MDM2 Q00987 |
P53 P04637 |
Cellular assay | EdU Cell Proliferation Assay | SJSA-1 cells | pIC50 (half maximal inhibitory concentration, -log10) | 6.37 |
| Ta | Structure | Name | Drugbank ID |
|---|---|---|---|
| 0.6287 | AMG-232 | DB15299 | |
| 0.5833 | Saquinavir | DB01232 | |
| 0.5746 | Yohimbine | DB01392 | |
| 0.5706 | CP-320626 | DB03383 | |
| 0.5600 | Cp403700, (S)-1-{2-[(5-Chloro-1h-Indole-2-Carbonyl)-Amino]-3-Phenyl-Propionyl}-Azetidine-3-Carboxylate | DB03744 | |
| 0.5548 | ABC-294640 | DB12764 | |
| 0.5464 | (7as,12ar,12bs)-1,2,3,4,7a,12,12a,12b-Octahydroindolo[2,3-a]Quinolizin-7(6h)-One | DB02191 | |
| 0.5460 | Osanetant | DB04872 | |
| 0.5400 | Metoserpate | DB11530 | |
| 0.5376 | Telinavir | DB12178 | |
| 0.5250 | 3-{6-[(8-HYDROXY-QUINOLINE-2-CARBONYL)-AMINO]-2-THIOPHEN-2-YL-HEXANOYLAMINO}-4-OXO-BUTYRI ACID | DB07916 | |
| 0.5052 | (1S,3R,6S)-4-oxo-6-{4-[(2-phenylquinolin-4-yl)methoxy]phenyl}-5-azaspiro[2.4]heptane-1-carboxylic acid | DB07189 | |
| 0.5047 | (4R)-7,8-dichloro-1',9-dimethyl-1-oxo-1,2,4,9-tetrahydrospiro[beta-carboline-3,4'-piperidine]-4-carbonitrile | DB07242 | |
| 0.5033 | Saredutant | DB06660 | |
| 0.4977 | Virginiamycin S1 | DB04805 |