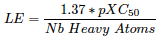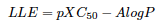Compound 2263
Identifiers
- Canonical SMILES:
CC=CCn1cc(-c2cccc(c2)C(=O)N(C)C)c2cc[nH]c2c1=O
- IUPAC name:
3-[6-(but-2-en-1-yl)-7-oxo-1H,6H,7H-pyrrolo[2,3-c]pyridin-4-yl]-N,N-dimethylbenzamide
- InChi:
InChI=1S/C20H21N3O2/c1-4-5-11-23-13-17(16-9-10-21-18(16)20(23)25)14-7-6-8-15(12-14)19(24)22(2)3/h4-10,12-13,21H,11H2,1-3H3
- InChiKey:
RJEMCUZKQLRUIS-UHFFFAOYSA-N
External links
 145767868 |
 69G |
External search

|

|

|
|

|
Bibliography (1)
Pharmacological data
| Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|
| 5 | 0 | 0 | 0 |
Targets
| PPI family | Best activity | Diseases | MMoA |
|---|---|---|---|
| Bromodomain / Histone | 6.80 | cancer | Inhibition |
Physicochemical filters
| Descriptor | Lipinski's RO5 | Veber | Pfizer's 3/75 | |
|---|---|---|---|---|
| Compliance | ||||
| MW | 335.16 g/mol | |||
| HBA | 5 | |||
| HBD | 1 | |||
| HBA + HBD | 6 | |||
| AlogP | 2.40 | |||
| TPSA | 56.41 | |||
| RB | 4 |
Radar chart
PCA : iPPI-DB chemical space
PCA : Correlation circle
Efficiencies: iPPI-DB biplot LE versus LLE
Summary
| Bibliographic ressources | Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|---|
| 1 | 5 | 0 | 0 | 0 |
Pharmacological data
| Bibliography | Name | Target | Competition | Assay type | Assay name | Cell line | Activity type | Activity |
|---|---|---|---|---|---|---|---|---|
| 10.1021/acs.jmedchem.6b00264 | Compound 4 | BRD4 O60885 |
H4 P62805 |
Biochemical assay | Time-Resolved FRET BRD4(1) | pIC50 (half maximal inhibitory concentration, -log10) | 6.34 | |
| 10.1021/acs.jmedchem.6b00264 | Compound 4 | BRD4 O60885 |
H4 P62805 |
Biochemical assay | Time-Resolved FRET BRD4(2) | pIC50 (half maximal inhibitory concentration, -log10) | 5.85 | |
| 10.1021/acs.jmedchem.6b00264 | Compound 4 | BRD4 O60885 |
H4 P62805 |
Biochemical assay | Time-Resolved FRET BRD9 | pIC50 (half maximal inhibitory concentration, -log10) | 6.80 | |
| 10.1021/acs.jmedchem.6b00264 | Compound 4 | BRD4 O60885 |
H4 P62805 |
Biochemical assay | Time-Resolved FRET TAF1(1) | pIC50 (half maximal inhibitory concentration, -log10) | 6.39 | |
| 10.1021/acs.jmedchem.6b00264 | Compound 4 | BRD4 O60885 |
H4 P62805 |
Biochemical assay | Time-Resolved FRET CECR2 | pIC50 (half maximal inhibitory concentration, -log10) | 5.24 |
| Ta | Structure | Name | Drugbank ID |
|---|---|---|---|
| 0.4767 | Ozenoxacin | DB12924 | |
| 0.4725 | 2-amino-5-[3-(1-ethyl-1H-pyrazol-5-yl)-1H-pyrrolo[2,3-b]pyridin-5-yl]-N,N-dimethylbenzamide | DB08583 | |
| 0.4706 | Dactolisib | DB11651 | |
| 0.4663 | N-(2-hydroxy-1,1-dimethylethyl)-1-methyl-3-(1H-pyrrolo[2,3-b]pyridin-2-yl)-1H-indole-5-carboxamide | DB06834 | |
| 0.4620 | 3-(2-aminoquinazolin-6-yl)-4-methyl-1-[3-(trifluoromethyl)phenyl]pyridin-2(1H)-one | DB07528 | |
| 0.4615 | 5-[3-(2-METHOXYPHENYL)-1H-PYRROLO[2,3-B]PYRIDIN-5-YL]-N,N-DIMETHYLPYRIDINE-3-CARBOXAMIDE | DB08350 | |
| 0.4557 | 2-Amino-1-methyl-6-phenylimidazo(4,5-b)pyridine | DB08398 | |
| 0.4521 | 4-(2-chlorophenyl)-8-(2-hydroxyethyl)-6-methylpyrrolo[3,4-e]indole-1,3(2H,6H)-dione | DB07257 | |
| 0.4444 | Perampanel | DB08883 | |
| 0.4444 | LY-3023414 | DB12167 | |
| 0.4431 | 3-(5-{[4-(AMINOMETHYL)PIPERIDIN-1-YL]METHYL}-1H-INDOL-2-YL)QUINOLIN-2(1H)-ONE | DB07025 | |
| 0.4372 | Tazemetostat | DB12887 | |
| 0.4364 | 4-(2-amino-1-methyl-1H-imidazo[4,5-b]pyridin-6-yl)phenol | DB06898 | |
| 0.4350 | 3-(9-HYDROXY-1,3-DIOXO-4-PHENYL-2,3-DIHYDROPYRROLO[3,4-C]CARBAZOL-6(1H)-YL)PROPANOIC ACID | DB07265 | |
| 0.4342 | Rosoxacin | DB00817 |