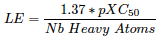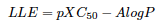Compound 2173
Identifiers
- Canonical SMILES:
Cc1cc(N=Nc2ccc(cc2)S(=O)(=O)Nc2ccccn2)c(N)c(Cl)c1O
- IUPAC name:
4-[2-(2-amino-3-chloro-4-hydroxy-5-methylphenyl)diazen-1-yl]-N-(pyridin-2-yl)benzene-1-sulfonamide
- InChi:
InChI=1S/C18H16ClN5O3S/c1-11-10-14(17(20)16(19)18(11)25)23-22-12-5-7-13(8-6-12)28(26,27)24-15-4-2-3-9-21-15/h2-10,25H,20H2,1H3,(H,21,24)
- InChiKey:
NPQKWPKFRWMJFA-UHFFFAOYSA-N
External links
 136226511 |
External search

|

|

|
|

|
Bibliography (1)
Pharmacological data
| Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|
| 4 | 2 | 0 | 1 |
Targets
| PPI family | Best activity | Diseases | MMoA |
|---|---|---|---|
| Bromodomain / Histone | 6.77 | cancer | Inhibition |
Physicochemical filters
| Descriptor | Lipinski's RO5 | Veber | Pfizer's 3/75 | |
|---|---|---|---|---|
| Compliance | ||||
| MW | 417.07 g/mol | |||
| HBA | 8 | |||
| HBD | 4 | |||
| HBA + HBD | 12 | |||
| AlogP | 4.23 | |||
| TPSA | 130.06 | |||
| RB | 4 |
Radar chart
PCA : iPPI-DB chemical space
PCA : Correlation circle
Efficiencies: iPPI-DB biplot LE versus LLE
Summary
| Bibliographic ressources | Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|---|
| 1 | 4 | 2 | 0 | 1 |
Pharmacological data
| Bibliography | Name | Target | Competition | Assay type | Assay name | Cell line | Activity type | Activity |
|---|---|---|---|---|---|---|---|---|
| 10.1021/jm401334s | Compound 29, MS363 | BRD4 O60885 |
H4 P62805 |
Biochemical assay | fluorescence anisotropy competition assay using fluorescein-labeled MS417 and BRD4 BrD1 | pKi (inhibition constant, -log10) | 6.77 | |
| 10.1021/jm401334s | Compound 29, MS363 | BRD4 O60885 |
H4 P62805 |
Biochemical assay | fluorescence anisotropy competition assay using fluorescein-labeled MS417 and BRD4 BrD2 | pKi (inhibition constant, -log10) | 6.19 | |
| 10.1021/jm401334s | Compound 29, MS363 | BRD4 O60885 |
H4 P62805 |
Biochemical assay | fluorescence anisotropy competition assay using fluorescein-labeled MS574 and BRD3 BrD1 | pKi (inhibition constant, -log10) | 6.62 | |
| 10.1021/jm401334s | Compound 29, MS363 | BRD4 O60885 |
H4 P62805 |
Biochemical assay | fluorescence anisotropy competition assay using fluorescein-labeled MS574 and BRD3 BrD2 | pKi (inhibition constant, -log10) | 6.49 | |
| 10.1021/jm401334s | Compound 29, MS363 | BRD4 O60885 |
H4 P62805 |
Cellular assay | Inhibition of BRD4 assessed as inhibition of NF-kappaB-mediated NO production | RAW264.7 cells | pIC50 (half maximal inhibitory concentration, -log10) | 5.47 |
| 10.1021/jm401334s | Compound 29, MS363 | BRD4 O60885 |
H4 P62805 |
Cellular assay | Inhibition of BRD4 assessed as inhibition of IL-6 expression measured by ELISA | RAW264.7 cells | pIC50 (half maximal inhibitory concentration, -log10) | 5.42 |
Cytotoxicity data
| Bibliography | Name | Assay name | Cell line | Compound concentration (μM) | Toxicity |
|---|---|---|---|---|---|
| 10.1021/jm401334s | Compound 29, MS363 | Cell Viability Study of Murine Macrophage Cells | Murinemacrophage RAW264.7 | 50.000 | no |
| Ta | Structure | Name | Drugbank ID |
|---|---|---|---|
| 0.7554 | Sulfasalazine | DB00795 | |
| 0.5935 | Sulfapyridine | DB00891 | |
| 0.5033 | Chlorsulfaquinoxaline | DB12921 | |
| 0.4932 | Sulfachlorpyridazine | DB11461 | |
| 0.4859 | 2-(6-{[(3-chloro-2-methylphenyl)sulfonyl]amino}pyridin-2-yl)-N,N-diethylacetamide | DB07056 | |
| 0.4789 | Sulfaquinoxaline | DB11464 | |
| 0.4658 | AMG-131 | DB05490 | |
| 0.4658 | Sulfabromomethazine | DB11547 | |
| 0.4643 | Sulfamethazine | DB01582 | |
| 0.4557 | N-{3-METHYL-5-[2-(PYRIDIN-4-YLAMINO)-ETHOXY]-PHENYL}-BENZENESULFONAMIDE | DB07944 | |
| 0.4552 | Sulfaperin | DB13320 | |
| 0.4500 | Sulfamerazine | DB01581 | |
| 0.4472 | Sulfaethoxypyridazine | DB11462 | |
| 0.4459 | Sulfamethoxypyridazine | DB13773 | |
| 0.4428 | Sulfadiazine | DB00359 |