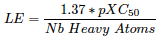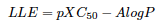Compound 1870
Identifiers
- Canonical SMILES:
Cc1[nH]c(-c2cc(NS(C)(=O)=O)ccc2Oc2ccccc2)c2cn[nH]c(=O)c12
- IUPAC name:
N-(3-{7-methyl-1-oxo-1H,2H,6H-pyrrolo[3,4-d]pyridazin-5-yl}-4-phenoxyphenyl)methanesulfonamide - InChi:
InChI=1S/C20H18N4O4S/c1-12-18-16(11-21-23-20(18)25)19(22-12)15-10-13(24-29(2,26)27)8-9-17(15)28-14-6-4-3-5-7-14/h3-11,22,24H,1-2H3,(H,23,25)
- InChiKey:
LPQIHHHQRDWFRN-UHFFFAOYSA-N
External links
 136387525 |
 CHEMBL4086135 |
 87M |
External search

|

|

|
|

|
Bibliography (1)
Pharmacological data
| Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|
| 1 | 2 | 0 | 0 |
Targets
| PPI family | Best activity | Diseases | MMoA |
|---|---|---|---|
| Bromodomain / Histone | 7.52 | cancer | Inhibition |
Physicochemical filters
| Descriptor | Lipinski's RO5 | Veber | Pfizer's 3/75 | |
|---|---|---|---|---|
| Compliance | ||||
| MW | 410.10 g/mol | |||
| HBA | 8 | |||
| HBD | 3 | |||
| HBA + HBD | 11 | |||
| AlogP | 1.71 | |||
| TPSA | 112.65 | |||
| RB | 4 |
Radar chart
PCA : iPPI-DB chemical space
PCA : Correlation circle
Efficiencies: iPPI-DB biplot LE versus LLE
Summary
| Bibliographic ressources | Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|---|
| 1 | 1 | 2 | 0 | 0 |
Pharmacological data
| Bibliography | Name | Target | Competition | Assay type | Assay name | Cell line | Activity type | Activity |
|---|---|---|---|---|---|---|---|---|
| 10.1016/j.bmcl.2017.02.057 | 28 | BRD4 O60885 |
H4 P62805 |
Biochemical assay | Time-Resolved FRET | pKi (inhibition constant, -log10) | 7.52 | |
| 10.1016/j.bmcl.2017.02.057 | 28 | BRD4 O60885 |
H4 P62805 |
Cellular assay | MX-1 proliferation assay | MX-1 | pEC50 (half maximal effective concentration, -log10) | 7.23 |
| 10.1016/j.bmcl.2017.02.057 | 28 | BRD4 O60885 |
H4 P62805 |
Cellular assay | Luciferase reporter | H1299 | pEC50 (half maximal effective concentration, -log10) | 6.74 |
| Ta | Structure | Name | Drugbank ID |
|---|---|---|---|
| 0.4467 | Dasabuvir | DB09183 | |
| 0.4042 | 3-(5-{[4-(AMINOMETHYL)PIPERIDIN-1-YL]METHYL}-1H-INDOL-2-YL)-1H-INDAZOLE-6-CARBONITRILE | DB07075 | |
| 0.4039 | [5-(5-Amino-1H-pyrrolo[3,2-b]pyridin-2-yl)-6-hydroxy-3'-nitro-3-biphenylyl]acetic acid | DB08232 | |
| 0.3956 | CRA_10433 | DB03173 | |
| 0.3911 | 4-[3-(1H-BENZIMIDAZOL-2-YL)-1H-INDAZOL-6-YL]-2-METHOXYPHENOL | DB07336 | |
| 0.3908 | 3-{5-[AMINO(IMINIO)METHYL]-1H-INDOL-2-YL}-5-METHOXY-1,1'-BIPHENYL-2-OLATE | DB07229 | |
| 0.3886 | CRA_10991 | DB01771 | |
| 0.3851 | [2'-HYDROXY-3'-(1H-PYRROLO[3,2-C]PYRIDIN-2-YL)-BIPHENYL-3-YLMETHYL]-UREA | DB07247 | |
| 0.3789 | 2-(2-Hydroxy-Phenyl)-1h-Indole-5-Carboxamidine | DB02463 | |
| 0.3750 | 6-(5-BROMO-2-HYDROXYPHENYL)-2-OXO-4-PHENYL-1,2-DIHYDROPYRIDINE-3-CARBONITRILE | DB08705 | |
| 0.3744 | ABT-072 | DB15156 | |
| 0.3739 | Tepotinib | DB15133 | |
| 0.3738 | (5-{3-[5-(PIPERIDIN-1-YLMETHYL)-1H-INDOL-2-YL]-1H-INDAZOL-6-YL}-2H-1,2,3-TRIAZOL-4-YL)METHANOL | DB07213 | |
| 0.3737 | 6-FLUORO-2-[2-HYDROXY-3-(2-METHYL-CYCLOHEXYLOXY)-PHENYL]-1H-INDOLE-5-CARBOXAMIDINE | DB06856 | |
| 0.3717 | Omipalisib | DB12703 |