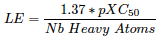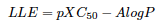Compound 1864
Identifiers
- Canonical SMILES:
Cc1[nH]c(c2CCNC(=O)c12)-c1ccccc1Oc1ccccc1
- IUPAC name:
3-methyl-1-(2-phenoxyphenyl)-2H,4H,5H,6H,7H-pyrrolo[3,4-c]pyridin-4-one
- InChi:
InChI=1S/C20H18N2O2/c1-13-18-16(11-12-21-20(18)23)19(22-13)15-9-5-6-10-17(15)24-14-7-3-2-4-8-14/h2-10,22H,11-12H2,1H3,(H,21,23)
- InChiKey:
QZKKZCIZIUWXFK-UHFFFAOYSA-N
External links
 86271752 |
 CHEMBL4104934 |
External search

|

|

|
|

|
Bibliography (1)
Pharmacological data
| Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|
| 1 | 2 | 0 | 0 |
Targets
| PPI family | Best activity | Diseases | MMoA |
|---|---|---|---|
| Bromodomain / Histone | 6.72 | cancer | Inhibition |
Physicochemical filters
| Descriptor | Lipinski's RO5 | Veber | Pfizer's 3/75 | |
|---|---|---|---|---|
| Compliance | ||||
| MW | 318.14 g/mol | |||
| HBA | 4 | |||
| HBD | 2 | |||
| HBA + HBD | 6 | |||
| AlogP | 3.43 | |||
| TPSA | 54.12 | |||
| RB | 3 |
Radar chart
PCA : iPPI-DB chemical space
PCA : Correlation circle
Efficiencies: iPPI-DB biplot LE versus LLE
Summary
| Bibliographic ressources | Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|---|
| 1 | 1 | 2 | 0 | 0 |
Pharmacological data
| Bibliography | Name | Target | Competition | Assay type | Assay name | Cell line | Activity type | Activity |
|---|---|---|---|---|---|---|---|---|
| 10.1016/j.bmcl.2017.02.057 | 22 | BRD4 O60885 |
H4 P62805 |
Biochemical assay | Time-Resolved FRET | pKi (inhibition constant, -log10) | 6.47 | |
| 10.1016/j.bmcl.2017.02.057 | 22 | BRD4 O60885 |
H4 P62805 |
Cellular assay | MX-1 proliferation assay | MX-1 | pEC50 (half maximal effective concentration, -log10) | 6.72 |
| 10.1016/j.bmcl.2017.02.057 | 22 | BRD4 O60885 |
H4 P62805 |
Cellular assay | Luciferase reporter | H1299 | pEC50 (half maximal effective concentration, -log10) | 6.55 |
| Ta | Structure | Name | Drugbank ID |
|---|---|---|---|
| 0.5864 | 2-[2-(2-FLUOROPHENYL)PYRIDIN-4-YL]-1,5,6,7-TETRAHYDRO-4H-PYRROLO[3,2-C]PYRIDIN-4-ONE | DB07728 | |
| 0.5628 | 2-(2-QUINOLIN-3-YLPYRIDIN-4-YL)-1,5,6,7-TETRAHYDRO-4H-PYRROLO[3,2-C]PYRIDIN-4-ONE | DB08358 | |
| 0.5340 | Rucaparib | DB12332 | |
| 0.5044 | N-[(13-CYCLOHEXYL-6,7-DIHYDROINDOLO[1,2-D][1,4]BENZOXAZEPIN-10-YL)CARBONYL]-2-METHYL-L-ALANINE | DB08031 | |
| 0.4974 | 9-HYDROXY-6-(3-HYDROXYPROPYL)-4-(2-METHOXYPHENYL)PYRROLO[3,4-C]CARBAZOLE-1,3(2H,6H)-DIONE | DB07006 | |
| 0.4821 | (7S)-2-(2-aminopyrimidin-4-yl)-7-(2-fluoroethyl)-1,5,6,7-tetrahydro-4H-pyrrolo[3,2-c]pyridin-4-one | DB07149 | |
| 0.4815 | Losmapimod | DB12270 | |
| 0.4802 | Atorvastatin | DB01076 | |
| 0.4652 | Talnetant | DB06429 | |
| 0.4531 | N-[(1R)-3-(4-HYDROXYPHENYL)-1-METHYLPROPYL]-2-(2-PHENYL-1H-INDOL-3-YL)ACETAMIDE | DB07991 | |
| 0.4476 | SLx-4090 | DB05678 | |
| 0.4413 | 3-(HYDROXYMETHYL)-1-METHYL-5-(2-METHYLAZIRIDIN-1-YL)-2-PHENYL-1H-INDOLE-4,7-DIONE | DB07385 | |
| 0.4358 | Esaxerenone | DB15207 | |
| 0.4348 | 1,3,4,9-Tetrahydro-2-(Hydroxybenzoyl)-9-[(4-Hydroxyphenyl)Methyl]-6-Methoxy-2h-Pyrido[3,4-B]Indole | DB04030 | |
| 0.4341 | 9-HYDROXY-4-PHENYL-6H-PYRROLO[3,4-C]CARBAZOLE-1,3-DIONE | DB04608 |