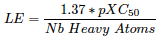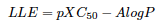Compound 1411
Identifiers
- Canonical SMILES:
CN[C@@H](C)C(=O)N[C@@H](COC)C(=O)N1CC[C@@H]2[C@H]1[C@H](CN2C(C)=O)c1c[nH]c2ccc(F)cc12
- IUPAC name:
(2S)-N-[(2S)-1-[(3aR,6S,6aR)-4-acetyl-6-(5-fluoro-1H-indol-3-yl)-2,3,3a,5,6,6a-hexahydropyrrolo[3,2-b]pyrrol-1-yl]-3-methoxy-1-oxopropan-2-yl]-2-(methylamino)propanamide
- InChi:
InChI=1S/C24H32FN5O4/c1-13(26-3)23(32)28-20(12-34-4)24(33)29-8-7-21-22(29)18(11-30(21)14(2)31)17-10-27-19-6-5-15(25)9-16(17)19/h5-6,9-10,13,18,20-22,26-27H,7-8,11-12H2,1-4H3,(H,28,32)/t13-,18+,20-,21+,22+/m0/s1
- InChiKey:
OZIABJPOLKJDSR-ACEXNVDFSA-N
External links
 45138703 |
External search

|

|

|
|

|
Bibliography (1)
| Publication | Name |
|---|---|
| Stephen M. Condon, Matthew G. Laporte, Tetralogic Pharmaceuticals Corp.. . Iap inhibitors None. | 72 |
Pharmacological data
| Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
Targets
| PPI family | Best activity | Diseases | MMoA |
|---|---|---|---|
| XIAP / Smac | 7.00 | cancer | Inhibition |
Physicochemical filters
| Descriptor | Lipinski's RO5 | Veber | Pfizer's 3/75 | |
|---|---|---|---|---|
| Compliance | ||||
| MW | 473.24 g/mol | |||
| HBA | 9 | |||
| HBD | 3 | |||
| HBA + HBD | 12 | |||
| AlogP | -0.45 | |||
| TPSA | 106.77 | |||
| RB | 7 |
Radar chart
PCA : iPPI-DB chemical space
PCA : Correlation circle
Efficiencies: iPPI-DB biplot LE versus LLE
Summary
| Bibliographic ressources | Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 0 |
Pharmacological data
| Bibliography | Name | Target | Competition | Assay type | Assay name | Cell line | Activity type | Activity |
|---|---|---|---|---|---|---|---|---|
| WO2010033531 | 72 | XIAP P98170 |
|
Biochemical assay | Fluorescence Polarization | pKd (dissociation constant, -log10) | 7.00 |
| Ta | Structure | Name | Drugbank ID |
|---|---|---|---|
| 0.7881 | Murepavadin | DB14777 | |
| 0.7632 | Somatoprim | DB12777 | |
| 0.7534 | Gramicidin D | DB00027 | |
| 0.7076 | Tifuvirtide | DB05413 | |
| 0.6962 | BQ-123 | DB12054 | |
| 0.6914 | Anamorelin | DB06645 | |
| 0.6842 | LTX-315 | DB12748 | |
| 0.6771 | Daptomycin | DB00080 | |
| 0.6763 | Nerofe | DB14786 | |
| 0.6725 | Abarelix | DB00106 | |
| 0.6627 | Acyline | DB11906 | |
| 0.6603 | Oglufanide | DB05779 | |
| 0.6595 | Somatostatin | DB09099 | |
| 0.6591 | Barusiban | DB12292 | |
| 0.6573 | Edratide | DB15272 |