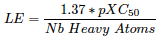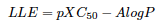Compound 1406
Identifiers
- Canonical SMILES:
CC[C@H](NC)C(=O)N[C@H]1[C@@H](CO)CC[C@H]2CC[C@H](N2C1=O)C(=O)NC(c1ccccc1)c1ccccc1
- IUPAC name:
(3S,6S,7S,9aS)-N-benzhydryl-7-(hydroxymethyl)-6-[[(2S)-2-(methylamino)butanoyl]amino]-5-oxo-1,2,3,6,7,8,9,9a-octahydropyrrolo[1,2-a]azepine-3-carboxamide
- InChi:
InChI=1S/C29H38N4O4/c1-3-23(30-2)27(35)32-26-21(18-34)14-15-22-16-17-24(33(22)29(26)37)28(36)31-25(19-10-6-4-7-11-19)20-12-8-5-9-13-20/h4-13,21-26,30,34H,3,14-18H2,1-2H3,(H,31,36)(H,32,35)/t21-,22+,23+,24+,26+/m1/s1
- InChiKey:
AYPTYGWZNLNCFL-VEGWMBEDSA-N
External links
 44237042 |
 CHEMBL573309 |
 24637608 |
External search

|

|

|

|

|
Bibliography (2)
Pharmacological data
| Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|
| 2 | 1 | 0 | 0 |
Targets
| PPI family | Best activity | Diseases | MMoA |
|---|---|---|---|
| XIAP / Smac | 6.64 | cancer | Inhibition |
Physicochemical filters
| Descriptor | Lipinski's RO5 | Veber | Pfizer's 3/75 | |
|---|---|---|---|---|
| Compliance | ||||
| MW | 506.29 g/mol | |||
| HBA | 8 | |||
| HBD | 4 | |||
| HBA + HBD | 12 | |||
| AlogP | 2.02 | |||
| TPSA | 110.77 | |||
| RB | 9 |
Radar chart
PCA : iPPI-DB chemical space
PCA : Correlation circle
Efficiencies: iPPI-DB biplot LE versus LLE
Summary
| Bibliographic ressources | Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|---|
| 2 | 2 | 1 | 0 | 0 |
Pharmacological data
| Bibliography | Name | Target | Competition | Assay type | Assay name | Cell line | Activity type | Activity |
|---|---|---|---|---|---|---|---|---|
| 19620011 | 3b | XIAP P98170 |
|
Biochemical assay | Fluorescence Polarization | pIC50 (half maximal inhibitory concentration, -log10) | 6.64 | |
| WO2009060292 | 24g | XIAP P98170 |
|
Biochemical assay | Fluorescence Polarization | pIC50 (half maximal inhibitory concentration, -log10) | 6.39 | |
| 19620011 | 3b | XIAP P98170 |
|
Cellular assay | Proliferation assay | MDA-MB-231 | pIC50 (half maximal inhibitory concentration, -log10) | 6.63 |
| Ta | Structure | Name | Drugbank ID |
|---|---|---|---|
| 0.6923 | N-cyclooctylglycyl-N-(4-carbamimidoylbenzyl)-L-prolinamide | DB06858 | |
| 0.6887 | 3-cyclohexyl-D-alanyl-N-(3-chlorobenzyl)-L-prolinamide | DB07190 | |
| 0.6857 | N-cycloheptylglycyl-N-(4-carbamimidoylbenzyl)-L-prolinamide | DB06853 | |
| 0.6857 | (S)-N-(4-carbamimidoylbenzyl)-1-(3-cyclopentylpropanoyl)pyrrolidine-2-carboxamide | DB07095 | |
| 0.6857 | (S)-N-(4-carbamimidoylbenzyl)-1-(3-cyclohexylpropanoyl)pyrrolidine-2-carboxamide | DB07131 | |
| 0.6822 | D-phenylalanyl-N-benzyl-L-prolinamide | DB07143 | |
| 0.6762 | (S)-N-(4-carbamimidoylbenzyl)-1-(2-(cyclohexylamino)ethanoyl)pyrrolidine-2-carboxamide | DB06850 | |
| 0.6731 | 1-[(2R)-2-aminobutanoyl]-N-(4-carbamimidoylbenzyl)-L-prolinamide | DB06947 | |
| 0.6731 | D-leucyl-N-(4-carbamimidoylbenzyl)-L-prolinamide | DB06996 | |
| 0.6727 | D-phenylalanyl-N-(3-methylbenzyl)-L-prolinamide | DB07133 | |
| 0.6604 | Proglumide | DB13431 | |
| 0.6571 | (S)-N-(4-carbamimidoylbenzyl)-1-(2-(cyclopentylamino)ethanoyl)pyrrolidine-2-carboxamide | DB06845 | |
| 0.6571 | 1-[(2R)-2-aminobutanoyl]-N-(3-chlorobenzyl)-L-prolinamide | DB06878 | |
| 0.6571 | D-leucyl-N-(3-chlorobenzyl)-L-prolinamide | DB06911 | |
| 0.6538 | 1-butanoyl-N-(4-carbamimidoylbenzyl)-L-prolinamide | DB06929 |