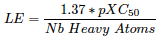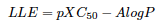Compound 1362
Identifiers
- Canonical SMILES:
CN1C(=O)N(C(=O)[C@]11CN(Cc2cc(cs2)C(O)=O)C[C@H]1c1ccc(cc1)C#N)c1cc(Cl)cc(Cl)c1
- IUPAC name:
5-[[(9R)-9-(4-cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]nonan-7-yl]methyl]thiophene-3-carboxylic acid
- InChi:
InChI=1S/C26H20Cl2N4O4S/c1-30-25(36)32(20-8-18(27)7-19(28)9-20)24(35)26(30)14-31(11-21-6-17(13-37-21)23(33)34)12-22(26)16-4-2-15(10-29)3-5-16/h2-9,13,22H,11-12,14H2,1H3,(H,33,34)/t22-,26+/m0/s1
- InChiKey:
NXNKJLOEGWSJGI-BKMJKUGQSA-N
External links
 11635371 |
 CHEMBL214529 |
 9810115 |
 2IC |
External search

|

|

|

|

|
Bibliography (1)
Pharmacological data
| Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
Targets
| PPI family | Best activity | Diseases | MMoA |
|---|---|---|---|
| LFA / ICAM | 7.70 | immune system disease | Inhibition |
Physicochemical filters
| Descriptor | Lipinski's RO5 | Veber | Pfizer's 3/75 | |
|---|---|---|---|---|
| Compliance | ||||
| MW | 554.06 g/mol | |||
| HBA | 8 | |||
| HBD | 1 | |||
| HBA + HBD | 9 | |||
| AlogP | 2.09 | |||
| TPSA | 104.95 | |||
| RB | 5 |
Radar chart
PCA : iPPI-DB chemical space
PCA : Correlation circle
Efficiencies: iPPI-DB biplot LE versus LLE
Summary
| Bibliographic ressources | Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 0 |
| Ta | Structure | Name | Drugbank ID |
|---|---|---|---|
| 0.5413 | Degarelix | DB06699 | |
| 0.4823 | Cp403700, (S)-1-{2-[(5-Chloro-1h-Indole-2-Carbonyl)-Amino]-3-Phenyl-Propionyl}-Azetidine-3-Carboxylate | DB03744 | |
| 0.4821 | Olcegepant | DB04869 | |
| 0.4791 | 2-CHLORO-N-[(3R)-2-OXO-1,2,3,4-TETRAHYDROQUINOLIN-3-YL]-6H-THIENO[2,3-B]PYRROLE-5-CARBOXAMIDE | DB07066 | |
| 0.4781 | (S)-2-CHLORO-N-(1-(2-(2-HYDROXYETHYLAMINO)-2-OXOETHYL)-2-OXO-1,2,3,4-TETRAHYDROQUINOLIN-3-YL)-6H-THIENO[2,3-B]PYRROLE-5-CARBOXAMIDE | DB07792 | |
| 0.4746 | Telinavir | DB12178 | |
| 0.4723 | Ibodutant | DB12042 | |
| 0.4688 | 5-Chloro-thiophene-2-carboxylic acid ((3S,4S)-1-{[2-fluoro-4-(2-oxo-2H-pyridin-1-yl)-phenylcarbamoyl]-methyl}-4-hydroxy-pyrrolidin-3-yl)-amide | DB07875 | |
| 0.4677 | 5-chloro-N-[(3R)-1-(2-{[2-fluoro-4-(2-oxopyridin-1(2H)-yl)phenyl]amino}-2-oxoethyl)pyrrolidin-3-yl]thiophene-2-carboxamide | DB07872 | |
| 0.4655 | CP-320626 | DB03383 | |
| 0.4609 | BMS-564929 | DB07286 | |
| 0.4588 | 5-CHLORO-THIOPHENE-2-CARBOXYLIC ACID ((3S,4S)-4-FLUORO- 1-{[2-FLUORO-4-(2-OXO-2H-PYRIDIN-1-YL)-PHENYLCARBAMOYL]-METHYL}-PYRROLIDIN-3-YL)-AMIDE | DB08143 | |
| 0.4558 | Bentiromide | DB00522 | |
| 0.4458 | Saquinavir | DB01232 | |
| 0.4397 | (20S)-19,20,21,22-TETRAHYDRO-19-OXO-5H-18,20-ETHANO-12,14-ETHENO-6,10-METHENO-18H-BENZ[D]IMIDAZO[4,3-K][1,6,9,12]OXATRIAZA-CYCLOOCTADECOSINE-9-CARBONITRILE | DB08674 |