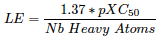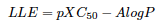Compound 1292
Identifiers
- Canonical SMILES:
OC(=O)[C@@H](N1[C@@H](c2ccc(Cl)cc2)C(=O)Nc2ccc(I)cc2C1=O)c1ccc(Cl)cc1
- IUPAC name:
(2S)-2-(4-chlorophenyl)-2-[(3S)-3-(4-chlorophenyl)-7-iodo-2,5-dioxo-2,3,4,5-tetrahydro-1H-1,4-benzodiazepin-4-yl]acetic acid
- InChi:
InChI=1S/C23H15Cl2IN2O4/c24-14-5-1-12(2-6-14)19-21(29)27-18-10-9-16(26)11-17(18)22(30)28(19)20(23(31)32)13-3-7-15(25)8-4-13/h1-11,19-20H,(H,27,29)(H,31,32)/t19-,20-/m0/s1
- InChiKey:
HQEQUYKKMMKSSX-PMACEKPBSA-N
External links
 656933 |
 CHEMBL361103 |
 571179 |
 DIZ |
External search

|

|

|

|

|
Bibliography (4)
Pharmacological data
| Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|
| 5 | 3 | 0 | 0 |
Targets
| PPI family | Best activity | Diseases | MMoA |
|---|---|---|---|
| MDM2-Like / P53 | 7.10 | cancer | Inhibition |
Physicochemical filters
| Descriptor | Lipinski's RO5 | Veber | Pfizer's 3/75 | |
|---|---|---|---|---|
| Compliance | ||||
| MW | 579.95 g/mol | |||
| HBA | 6 | |||
| HBD | 2 | |||
| HBA + HBD | 8 | |||
| AlogP | 6.35 | |||
| TPSA | 89.54 | |||
| RB | 4 |
Radar chart
PCA : iPPI-DB chemical space
PCA : Correlation circle
Efficiencies: iPPI-DB biplot LE versus LLE
Summary
| Bibliographic ressources | Biochemical tests | Cellular tests | PK tests | Cytotoxicity tests |
|---|---|---|---|---|
| 4 | 5 | 3 | 0 | 0 |
Pharmacological data
| Bibliography | Name | Target | Competition | Assay type | Assay name | Cell line | Activity type | Activity |
|---|---|---|---|---|---|---|---|---|
| 15664854 | 20 | MDM2 Q00987 |
|
Biochemical assay | Fluorescence Polarization | pIC50 (half maximal inhibitory concentration, -log10) | 6.38 | |
| 16600594 | 1 | MDM2 Q00987 |
|
Biochemical assay | Fluorescence Polarization | pIC50 (half maximal inhibitory concentration, -log10) | 6.38 | |
| 15715460 | 1 | MDM2 Q00987 |
|
Biochemical assay | Fluorescence Polarization | pKd (dissociation constant, -log10) | 7.10 | |
| 16600594 | 1 | MDM2 Q00987 |
|
Cellular assay | Proliferation assay | MCF7 mammary carcinoma cells | pIC50 (half maximal inhibitory concentration, -log10) | 4.42 |
| 16600594 | 1 | MDM2 Q00987 |
|
Cellular assay | Proliferation assay | MDA MB321 mammary carcinoma cells | pIC50 (half maximal inhibitory concentration, -log10) | 3.89 |
| 10.1021/jm049137g | 1 | MDM2 Q00987 |
P53 P04637 |
Cellular assay | Proliferation assay | HTB-144 | pIC50 (half maximal inhibitory concentration, -log10) | 4.52 |
| 10.1021/jm049137g | 1 | MDM2 Q00987 |
P53 P04637 |
Biochemical assay | Fluorescence Polarization | pIC50 (half maximal inhibitory concentration, -log10) | 6.70 | |
| 10.1021/jm049137g | 1 | MDM2 Q00987 |
P53 P04637 |
Biochemical assay | Fluorescence Polarization | pKd (dissociation constant, -log10) | 7.10 |
| Ta | Structure | Name | Drugbank ID |
|---|---|---|---|
| 0.5350 | Fominoben | DB08968 | |
| 0.4940 | Bentiromide | DB00522 | |
| 0.4822 | Oxazolam | DB15491 | |
| 0.4753 | [(2S)-4-methyl-3-oxo-2,3,4,5-tetrahydro-1H-1,4-benzodiazepin-2-yl]acetic acid | DB08717 | |
| 0.4746 | Ioxaglic acid | DB09313 | |
| 0.4695 | [1-(3-CHLORO-2-FORMYL-PHENYLCARBAMOYL)-2-METHYL-PROPYL]-CARBAMIC ACID TERT-BUTYL ESTER | DB07956 | |
| 0.4606 | 2-{[N-(2-ACETYL-5-CHLORO-4-FLUOROPHENYL)GLYCYL]AMINO}BENZOIC ACID | DB07085 | |
| 0.4575 | Evocalcet | DB12388 | |
| 0.4540 | ALPHA-(2,6-DICHLOROPHENYL)-ALPHA-(2-ACETYL-5-METHYLANILINO)ACETAMIDE | DB07332 | |
| 0.4510 | Cloxazolam | DB01553 | |
| 0.4503 | Indoprofen | DB08951 | |
| 0.4471 | (1S)-1-(3-chlorophenyl)-2-oxo-2-[(1,3,4-trioxo-1,2,3,4-tetrahydroisoquinolin-5-yl)amino]ethyl acetate | DB08498 | |
| 0.4410 | Dibenzepin | DB13225 | |
| 0.4381 | RG-4733 | DB11870 | |
| 0.4378 | (1S)-2-oxo-1-phenyl-2-[(1,3,4-trioxo-1,2,3,4-tetrahydroisoquinolin-5-yl)amino]ethyl acetate | DB08497 |